Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Protective Effects of Stem Cell-Derived Peptides in Preventing Autoimmune Diabetes in the Non-Diabetic Mouse Model
*Corresponding author: Jonathan RT Lakey, Professor Emeritus, Departments of Surgery and Biomedical Engineering, University of California Irvine, CA, USA.
Received: May 17, 2022; Published: June 29, 2022
DOI: 10.34297/AJBSR.2022.16.002264
Abstract
Type 1 Diabetes Mellitus (T1DM) is an important autoimmune disease characterized by the destruction of insulin-secreting beta cells in pancreatic islets of Langerhans. A deficiency in insulin level results in hyperglycemia in diabetic patients. The management of T1DM requires lifelong daily injections of exogenous insulin to control blood sugar levels. The pathophysiology of Diabetes Mellitus (DM), including TIDM, is a foundational disease model for autoimmune diseases in humans and other mammals. This study aimed to investigate the protective effect of Mito Organelle (MO) Peptides, a mixture of organ-specific cellular extracts that are extracted from organ specific cells, homogenized, filtered, and sterilized, on the function and survival of peptides on pancreatic beta cells involved in T1DM. Non-Obese Diabetes (NOD) mice models were used to determine the therapeutic intervention effect of MO peptides as they develop spontaneous and accelerated diabetes. In our study, we analyzed the effects of twice-weekly injections of BioPep MO peptides over 17 weeks in NOD mice on the progression of autoimmune-mediated beta-cell destruction and the onset of hyperglycemia. It was found that NOD MO treated mice had a lower blood glucose concentration than NOD saline treated mice on average. In conjunction, at the end of week 17, there was a 33% larger non-diabetic population within the MO peptide treated group versus the saline treated group. These studies will help understand the mechanisms of immunological protection in T1DM and may serve as a model for other autoimmune disorders.
Keywords: Peptides, Diabetes, Prevention of Diabetes, Autoimmune
Introduction
Type 1 Diabetes Mellitus (T1DM) is a chronic autoimmune disease that results from the destruction of the insulin-producing beta cells in pancreatic islets of Langerhans [1-3]. Insulin is an anabolic hormone that is essential to the regulation of blood glucose levels, and it exerts multiple effects on lipid, protein, glucose, and mineral metabolism. Critically, insulin allows for transportation of glucose into the cells and their mitochondria, predominantly in brain, heart, liver, and muscles. Also, insulin stimulates the uptake of amino acids, stimulates the synthesis of fatty acids and stores glucose in the liver, and inhibits the breakdown of fat in adipose tissue. In the development of T1DM in humans, the immune system destroys the beta cells in the pancreatic islets over the course of months or years, causing an absolute deficiency of insulin [1-3]. A deficiency in the production of insulin can lead to hyperglycemia, which when left untreated, can lead to long-term complications such as kidney disease, cardiovascular disease, peripheral arterial disease, stroke, and other diabetic-related complications. At present, there is no cure for T1DM, and current treatments focus on the management of blood glucose levels through the lifetime administration of exogenous insulin.
Peptide therapy aims to either induce peptide production or reinstate normal signaling patterns by renewing the strength of the signals received by cells [4-6]. Due to the differences in peptides produced between different tissue types, peptide therapy utilizes organ-specific extracts to target aging or diseased tissue with the goal of revitalizing normal peptidergic signaling in these regions. Preserving an individual’s endogenous insulin secretion through the modulation of the immune system has been proposed as an optimal solution for those with diabetogenic T cells [7]. Modulating the pathogenic T-cell response with antigen-specific peptide immunotherapy may offer the potential for preventing further tissue destruction and restoring immune homeostasis. Peptide immunotherapy offers the potential to prevent the immunemediated response on the beta cells and opens the possibility for beta-cell regeneration or replacement therapies to become more effective and long-lasting.
T1DM represents an ideal autoimmune disease for trialing peptide therapy due to our extensive knowledge of the etiology and pathology of the disease and the presence of the Non-Obese Diabetic (NOD) mouse model that parallels the disease course in humans [8]. The NOD mouse model is widely used for T1DM research due to its natural development of diabetogenic T cells that lead to the destruction of the beta cells of the pancreatic islets of Langerhans and result in insulin deficiency [9]. As early as 3 weeks of age, the innate immune cells of NOD mice infiltrate the islets of the pancreas and trigger the response of the adaptive immune system. As the NOD mice age, autoreactive T cells that recognize specific diabetes-related autoantigens begin to develop within the thymus and escape negative selection into the periphery [9]. Once in the periphery, these T cells fail to be controlled and instead become activated, leading to the destruction of the insulin-producing beta cells in the pancreatic islets of Langerhans. In addition to paralleling the spontaneous development of T1DM as in humans, NOD mice also exhibit similar symptoms to diabetic humans such as glycosuria, polydipsia, weight loss, and polyuria [10-13]. A prior study [14] found that the administration of mitochondrial-encoded peptides in NOD mice could modulate T cells and induce changes in the function and phenotype of the immune cells. These results indicated a significant decrease in the incidence of diabetes in NOD mice and the reduction of T cell activation, supporting the notion that peptide therapy may ameliorate the progression or prevent the onset of T1DM in humans.
BioPep Peptides Used in this Study
European Wellness (EW) and the BioPep Research Group have developed a peptide therapy product made of organ-specific cellular extracts and peptide molecules that are intended for therapeutic use in both animals and humans [15-24]. EW and BioPep manufacture two distinct peptide products, Mito Organelles (MO) peptides and Nano Organo Peptides (NOP), which are produced through a proprietary parallel-extraction process from mammalian precursor stem cells and rabbits bred in closed colonies under good manufacturing practices conditions. MO peptides are biologically extracted mixtures of cellular peptides that have predominantly mitochondria-specific functions [25]. Unlike NOPs, which are under 10kDa in size due to their extraction process, MOs are larger in size and have predominantly mitochondria-specific functions allowing for a more pronounced revitalization of mitochondrial deficits. MO peptides are organ-specific extracts that aim to revitalize and rejuvenate mitochondrial activity, thereby regenerating diseased or aging tissue.
These Mitochondria-Derived Peptides (MDPs) are widely distributed through various tissues and have been shown to play cryoprotective roles through maintaining cell viability and mitochondrial function under both pathological and normal conditions [26,27]. This study seeks to determine whether the administration of stem-cell derived MO peptides twice-weekly to NOD mice through intramuscular injections over 17 weeks delays or prevents the onset of the destruction of the insulin-secreting beta cells in pancreatic islets of Langerhans. Specifically, the stemcell derived MO peptides utilized in this study will be obtained from thymus and pancreatic extracts to target the regions of the beta cells and T-cell maturation [1,28]. It is hypothesized mice administered with MO peptides will have better maintained blood glucose levels (mg/dL) throughout the study and be less susceptible to the development of T1DM as compared to those injected with saline alone. Evidence of a positive effect on survival and function of MO peptides on beta cells would provide important evidence for the protective effect of MO peptides on delaying hyperglycemia and diabetic onset.
Materials and Methods
This study was conducted under protocols reviewed and approved by the Institutional Animal Care and Use Committee at University of California Irvine, Protocol # AUP-17-241. The sample size included 12 female NOD mice (Charles River, San Diego, CA) which were purchased at an age of 6 weeks. Once acclimated to the facility, the mice were divided into MO peptide and saline injection groups. The selected concentration of MO peptides for administration was based on the human injection dose scaled down to the body weight of an average mouse (approximately 30-32g). The regular human dose of MO peptides is 7mcg/kg (0.035mL/ kg), and since each MO dose consists of approximately 500mcg, the dilution for the mice was scaled accordingly. Per weekly dose, 100 μL of MO peptide solution was diluted with 10mL of saline to achieve the same concentration used in humans. Mice received either 90μL of the diluted MO peptide solution or 90μL of saline solution as a control administered subcutaneously twice a week. The first injection of MO peptide or saline solution was administered at week 1 and the last injection was administered at week 17.
Blood Glucose Monitoring
Blood glucose measurements were obtained once a week in all mice using the tail capillary prick method. At week 1, nonfasting blood sugar levels were recorded immediately before the administration of the first dose of peptide or saline. Therefore, week 1 blood glucose readings represent the NOD mice basal glucose level and week 2 values indicate changes in blood glucose a week after the first round of injection. No injection and data collection were performed during weeks 8 and 9 as the closure of our animal facility (due to COVID) paused the experimental procedure for 2 weeks. The study resumed at week 10 and continued until week 13. For mice that had a blood glucose concentration ≥300mg/ dL, a follow up measurement was taken 2 days later to confirm transition from non-diabetic to diabetic. If the blood glucose concentration remained ≥300mg/dL, the mouse was deemed diabetic and sacrificed. An average of the two measurements was then used for the results in that given week. Mice were monitored for a total of 17 weeks unless they were deemed to be diabetic with three consecutive non fasting blood glucose readings from capillary blood collected from the tail vein and read using a commercial blood glucose meter (Abbott).
Serum Sample Collection for Cytokine Analysis
At the conclusion of the study or when mice became diabetic, the NOD mice in both groups were euthanized, blood samples were collected from each mouse utilizing a heart puncture method and serum samples were obtained. A cytokine panel (Mouse Cytokine 44-Plex Discovery Assay®, Eve Technologies) was performed on the collected serum samples for comparison of the cytokine levels between the MO peptide and saline groups.
Statistical Analysis
Data analysis and graph construction were performed in Microsoft Excel®. A two-sample t-test method was selected to compare the weekly blood glucose levels of the MO peptide and saline mice. Statistical testing was performed to compare blood sugars between the two groups at each week from week 2 until week 17. Statistical testing was also performed to compare week 1 blood sugar levels between the NOD saline and NOD MO mice to ensure groups were representative of each other. A probability of <0.05 was statistically significant.
Results
Baseline measurements of blood glucose concentration in NOD MO mice and saline mice were collected at week 1 (Figure 1). No statistical difference in the blood glucose concentration was found between the groups (p = 0.532). The blood glucose level of the MO peptide group was significantly lower than the saline group from week 2 to week 6 (p <0.05, paired t test. During weeks 7-8, there was a closure of the animal facility housing the NOD mice which paused the study progress for 2 weeks and no data was collected. The injections and blood sugar monitoring resumed at week 9 and no significant difference was found in the blood glucose levels of the two groups on weeks 9 (p = 0.327), 10 (p = 0.702), 11 (p = 0.867), 12 (p = 0.508), 13 (p = 0.110), 14 (p = 0.253), 15 (p = 0.376), 16 (p = 0.329), and 17 (p = 0.257). Although the blood glucose concentration of MO peptide and saline groups were not significantly different from week 10 to 13, the mean blood sugar in the MO peptide group began to drop on week 11 until the conclusion of the experiment at week 17 (Table 1).

Table 1: Mean weekly blood glucose concentrations (mg/dl) between NOD MO peptide mice and controls. Mice that were deemed diabetic (two consecutive readings ≥300mg/dL) were not included in the mean weekly blood glucose concentration (mg/dL) calculation following their sacrifice.
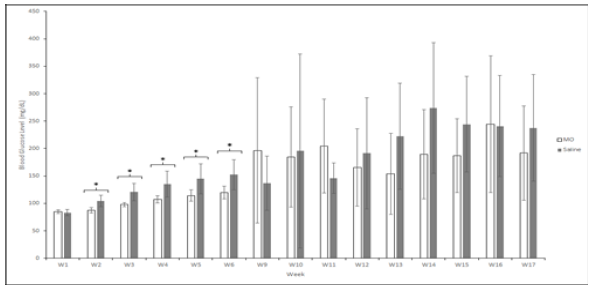
Figure 1: The change in the mean blood glucose concentration (mg/dL) of NOD mice in the MO peptide (n = 6) and control group (n = 6) over 17 weeks. Week 1 values represent the mean blood glucose level before the first injection of MO peptide or saline. Following the sacrifice of mice that were deemed diabetic (two consecutive readings ≥300d dd mg/dL), a blood glucose concentration of 300mg/dL was substituted for all subsequent weeks to provide a more accurate comparison of groups. Statistical analysis was performed after the first injection to compare the blood glucose concentration between the MO peptide and saline group each week. * Represents a significant difference (p < 0.0500). Error bars are mean ± SEM.
All mice in both MO peptide and saline groups were nondiabetic up until week 9 (Figure 2). Four out of the six MO treated mice remained non-diabetic at the end of the study, while only two of the six control mice remained non-diabetic. Within both groups, mice which developed diabetes (blood glucose >300mg/dL) while the study were sacrificed, and blood glucose measurements were continued only for non-diabetic mice. Although the percent of nondiabetic mice in the MO group showed an initial decrease at week 9 relative to the control mice, the control mice showed an overall greater decrease in the proportion of non-diabetic mice by week 17 of the study. There was a 33% greater non-diabetic population in the MO peptide treated mice versus the saline treated mice by the end of week 17.
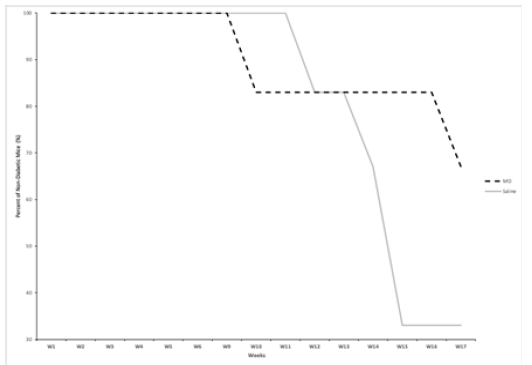
Figure 2: The change in percentage of non-diabetic NOD mice in the MO peptide (n=6) and control groups (n=6) over the course of 17 weeks. All mice that had two consecutive blood glucose readings ≥300mg/dL were sacrificed and were no longer included in data recordings.
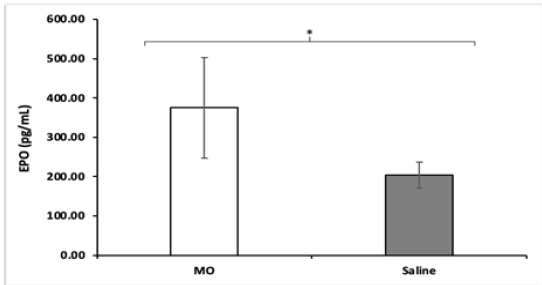
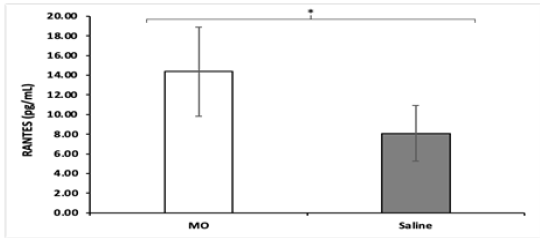
All NOD mice were sacrificed at the end of the study and a cytokine panel was obtained using serum samples of each mouse. The panel consisted of 45 cytokine assays, and a significant difference in the concentration of Erythropoietin (EPO) and Chemokine Ligand 5 (CCL5), otherwise known as RANTES, was found between MO peptide mice and control mice. NOD MO peptide mice had an average EPO concentration of 374.88pg/mL while the NOD saline mice had an average EPO concentration of 203.68pg/ mL (Figure 3). The differences in EPO concentrations are higher (p = 0.0062) in the MO peptide treated group compared to the saline treated ones. Likewise, NOD MO peptide mice had an average CCL5 concentration of 14.37pg/mL while the NOD saline mice had an average EPO concentration of 8.08pg/mL (Figure 4). The differences in CCL5 concentrations were statistically significantly higher in the MO peptide treated group compared to the controls (p = 0.031).
Discussion
T1DM is an autoimmune disease characterized by the destruction of insulin-secreting beta cells in the pancreatic islets of Langerhans [1-3], with no current cure. The NOD mouse has played a critical role in strengthening our understanding of T1DM by permitting researchers to conduct a variety of studies that be otherwise impossible [29]. The administration of exogenous peptides, known as peptide therapy, aims to either induce peptide production or reinstate normal signaling patterns by renewing the strength of the signals received by cells. Due to the differences in peptides produced between different tissue types, peptide therapy utilizes organ-specific extracts to target aging or diseased tissue with the goal of revitalizing normal peptidergic signaling in these cells and improving overall health. Prior research has demonstrated the effectiveness of peptide therapy in decreasing the incidence of diabetes in NOD mice and reducing T cell activation [18].
Our study sought to investigate whether the administration of thymus and pancreatic MO peptides over the course of 17 weeks would affect the development of T1DM. MO peptides are mitochondrial derived organ specific peptides produced by European Wellness and BioPep Research Group through a proprietary parallel-extraction process. All mice in both MO peptide and control groups were non-diabetic up until week 9 (Figure 2). Four out of the six MO treated mice remained non-diabetic at the end of the study while only two of the six controls mice remained non-diabetic. Although the percent of non-diabetic mice in the MO group had an initial decrease at week 9 relative to the saline mice, the mice in the saline group showed an overall greater decrease in percent of non-diabetics by week 17 of the study. Results showed a 33% greater non-diabetic population in the MO peptide treated mice versus the saline treated mice by the end of week 17. At the conclusion of the study on week 17, all remaining NOD mice were sacrificed for cytokine analysis.
The panel consisted of 45 cytokines and the assay revealed two cytokines, EPO and CCL5, that were much more prevalent in the MO peptide mice. EPO is a glycoprotein that regulates erythrocyte production; specifically involved in stimulating proliferation and differentiation of erythrocytes as well as preventing apoptosis in these cells [30]. The chemokine CCL5, also known as RANTES (regulation on activation, normal T cell expressed and secreted) is a proinflammatory cytokine that recruits leukocytes to the site of inflammation [31-33]. Together, these results indicate that an underlying change to the signaling and physiology of the NOD mice occurred. These changes may have protected from the destruction of the beta pancreatic cells of Langerhans and resulted in the delayed onset or possible prevention of diabetes in the treatment group. However, it’s important to note the small sample size and relatively short study time. Further research is necessary in longer and larger studies, as well as to determine the exact mechanism/s by which this protective effect occurred.
Conclusion
T1DM serves as a foundational disease model for autoimmune diseases in mammals and humans due to our extensive knowledge of the pathophysiology and etiology of T1DM and the presence of the NOD model mouse that closely parallels the disease course of humans. This pilot study demonstrates the beneficial effects of intramuscular delivery of stem cell derived peptides in delaying the onset of autoimmune diabetes in NOD mice. It provides important preliminary data that suggests that MO peptides may assist in delaying the onset or preventing T1DM and represents an exciting therapeutic option to further investigate. Future studies will focus on timing of delivery of organ specific stem cell peptides and further investigate the dose of peptide and duration of effect.
Funding
This project was funded by a research grant from BioPep Inc to the University of California Irvine.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.